Guide to UV Measurement
Significance of Absorbance
The ability to penetrate a film with light may be thought of in terms of “optical thickness” of the film – a combination of the physical thickness of the film and its absorptivity. It may also be expressed as a ratio of the light flux through the top of the film to the light flux through the bottom. This will aid in analyzing the cure condition as well as explaining depth of cure and adhesion behavior.
The reduction of radiant energy as it passes through any material is described by the Beer-Lambert law:
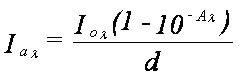
where Io is the incident energy, Ia is the energy absorbed, Aλ is the absorbance, all with respect to some specific wavelength λ, and d is the depth from the surface or film thickness.
Energy that is not absorbed in an upper layer of the film (and not reflected or scattered) is transmitted and available to lower layers. The optical properties of a coating help determine the selection of a photoinitiator.
To examine the significance of this equation, a film is divided into 100 “layers” (each 1% of the physical thickness) to reveal the relative absorption in the top surface (1% layer) and the extreme bottom (1% layer) of the film as a function of absorbance.
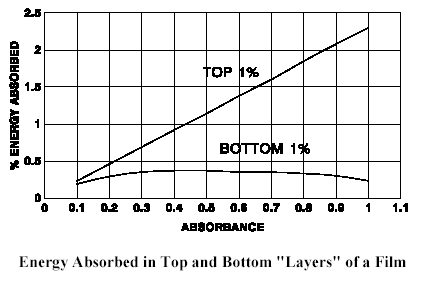
Calculations show that the optimum absorbance at a given wavelength by a film of any thickness is about 0.43. In other words, the maximum absorption in the bottom layer occurs when there is a “best” combination of film thickness and absorption. This also illustrates that even at the best conditions, energy absorbed at the top surface is 2 to 3 times the energy absorbed at the bottom. For a film such as a paint or an ink with an absorbance of three or more, this ratio will exceed one thousand!