Guide to UV Measurement
Photoinitiator Selection
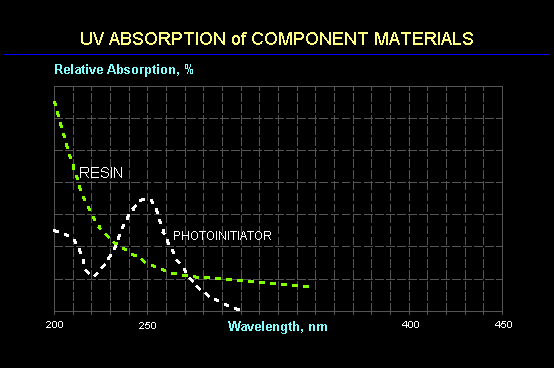
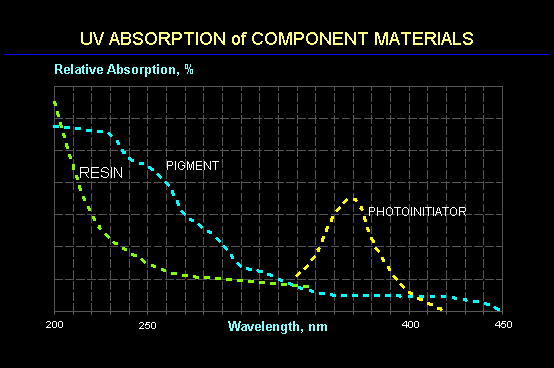
The charts below show typical spectral absorption curves for a photoinitiator, pigment and prepolymer (resin). In general, absorption of UV energy by an organic molecule increases as the wavelength decreases. Since the photoinitiator is designed to have an enhanced absorption of UV energy, it is not surprising that the wavelengths that are absorbed strongly are blocked from penetrating to deeper photoinitiator molecules. As a result, short UV wavelengths (200-280 nm) tend to be absorbed at or near the surface and are not available at lower depths. This leads to a limit in the film thickness that can be cured using only short wavelength UV with adhesion to the substrate often being the first property to suffer.
It is for this reason that most UV curing is accomplished using broadband sources having spectral energy distributions spanning a wide range of wavelengths. Thus, while the absorbance (or optical density) of a coating at short wavelengths may be significantly larger than the optimal value of 0.43, the absorbance at longer wavelengths can be significantly less. It is for this reason that a photoinitiator that may be appropriate for a clear coating or for a thin film may not be an appropriate selection for an ink where a photoinitiator with a longer wavelength response would be a better choice.