Guide to UV Measurement
Free Radical Polymerization
Acrylates and methacrylates make up the largest class of free-radically polymerizable resins. When exposed to a UV source, the photoinitiator decomposes to generate one or more radical species R• that can add to the unsaturated double bond of a (meth)acrylate monomer or oligomer to generate a new radical RM• and so on. Chain termination occurs by radical-radical recombination. Molecular oxygen (O2) is also an efficient scavenger of radicals and steps must be taken to either eliminate oxygen by inerting or overcoming oxygen diffusion by generating very high local radical concentrations. The latter is especially important for obtaining surface cure of free radical chemistries in air. A simplistic mechanism for a free radical polymerization process is shown below:
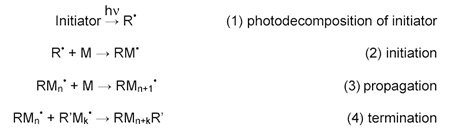
To learn about cationic polymerizations, click here.