Guide to UV Measurement
Cationic Polymerization
Examples of chemistries that polymerize via a cationic mechanism include epoxies and vinyl ethers. There is no equivalent of an inherent termination reaction for cationic systems since they are positively charged and repel each other. Cationic polymerizations are typically much slower than free radical polymerizations. For this reason, a high-speed process involving a cationic cure mechanism will often include some means to post-heat to complete the cure in the absence of the UV source. This dark reaction is a normal part of the polymerization process. Cationic systems are susceptible to water and base contamination. A simplified mechanism follows:
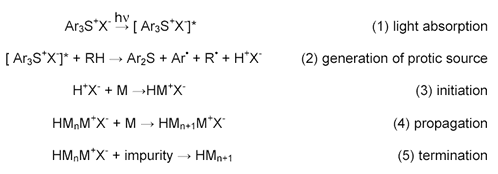
To learn about free radical polymerizations, click here.