Guide to UV Measurement
Photoinitiator
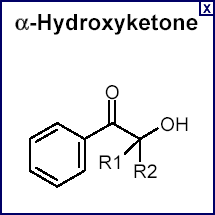
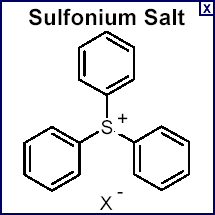
The photoinitiator is a critical element of the UV curing process. Photoinitiator is blended with a reactive chemistry to create a UV-curable formulation such as an ink or coating. The photoinitiator is responsible for initiating the chemical reaction by converting the energy from a UV source into a free radical or cationic initiating species. This initiating species causes the reactive chemistry to polymerize to give a cured ink or coating. Examples of common photoinitiators for free radical polymerizations are ![]() hydroxyketones and benzophenone while iodonium and sulfonium salts are examples of cationic initiators.
hydroxyketones and benzophenone while iodonium and sulfonium salts are examples of cationic initiators.

In order for a photoinitiator to start the polymerization process, it must first absorb UV energy from a UV source. For this reason, it is desirable to know both the spectral energy distribution of the source as well as the absorption spectrum of the photoinitiator. The absorption spectrum of a specific photoinitiator can usually be obtained directly from the supplier's literature. Alternatively, it is easily measured using a UV-visible spectrophotometer. The absorption spectrum of a photoinitiator relates to the probability of energy absorption at each wavelength and hence, to the probability that initiation will occur. The spectrum shown above is for a generic ![]() hydroxyketone .
hydroxyketone .
Unfortunately, seldom is the photoinitiator the only species in a formulation capable of absorbing UV energy. Normally, there is a competition between multiple absorbers and identifying a wavelength window where significant energy will be absorbed by the photoinitiator is critical. For this reason, a key part of proper formulating is selection of an appropriate photoinitiator. For more information on selecting a photoinitiator, click here. To learn more about absorbance and the absorption process, click here.