Guide to UV Measurement
UV Source
The final element of the UV curing process is a source of radiant energy in a spectral region appropriate for the photoinitiator selected. In most cases, the energy required will lie in the UV region of the electromagnetic spectrum and can come from a natural source such as the sun or an artificial source such as a medium pressure mercury lamp.
In sources containing mercury, the UV energy results from a radiative decay process of excited mercury atoms found in the gas plasma within the bulb. The energy of the emitted radiation is characteristic of mercury vapor and, while rich in UV, typically contains significant visible and infrared radiation as well. The actual energy distribution is dependent on a number of parameters including internal bulb pressure and bulb temperature. The spectral energy distribution of a medium pressure mercury lamp, the most common lamp found in UV processes is shown below:
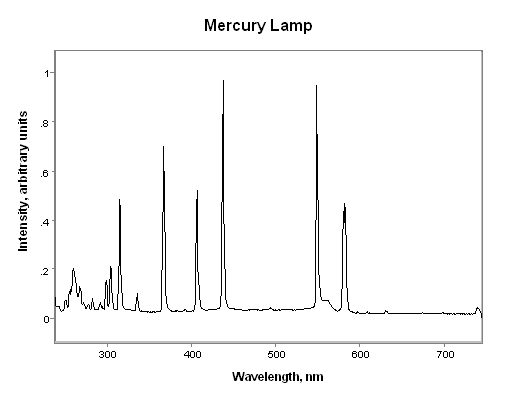
There are several other bulbs that have different spectral energy distributions that are closely related to a medium pressure mercury lamp. Small amounts of certain metal salts when present inside a medium pressure mercury bulb have a dramatic impact on the bulb's output. Both iron and gallium additive bulbs are available today from most lamp manufacturers.
The medium pressure mercury lamp is a broadband source in that its radiant energy occurs over a wide range of wavelengths. For convenience, the UV spectral region is broken up into smaller regions in much the same way that visible light is broken up into red, orange, yellow, etc. In the UV region, however, there are no simple descriptors that adequately identify the regions so the terminology used is simply UVA, UVB, and UVC. These refer to the long wave UV, the mid-range UV, and the short wave UV regions, respectively. In the same way that there is no clear dividing wavelength between red and orange visible light, the divisions between the different UV regions is also somewhat arbitrary. As a general guideline, the UVA region spans from 315-400 nm, the UVB region from 280-315 nm, and the UVC region from 100-280 nm. Note that the entire UV region spans from 100-400 nm but below 200 nm, the UV energy is readily absorbed by air and this region is seldom used for UV processes. As a result, most people involved in UV curing refer to the wavelength range from 200-400 nm as the UV region of the spectrum. For more information on UV band designations, click here.
Visible light can also be used to initiate a �UV� curing process. Use of visible light sources requires that the photoinitiator have some absorption in the visible region of the spectrum and, as a result, implies use of a starting formulation that has some inherent color and that may be unstable when exposed to room or sunlight. Even though these systems may require special ambient lighting and handling, however, they are an industrially important class.
Regardless of the source of the energy, when a UV-curable formulation is exposed to energy that overlaps with the absorption spectrum of the photoinitiator present, there is a finite probability that some amount of the energy will be absorbed by the photoinitiator and an initiating species will be generated. Once generated, the initiating species act as a trigger to turn on a chemical reaction within the reactive chemistry.
A UV source is actually much more than �just a light�. Sources today are sophisticated machines that can vary greatly in design. It is important to know what is right for your application. The ideal source is one that can provide a stable stream of photons having the proper spectral energy distribution to interact efficiently with the chemistry to be cured. To learn more about the different components that make up a UV source and how they can affect cure, click here.